Synthesis of Ni(Co)/Pd Ternary Nanostructures and Their Catalytic Activity in p–Nitrophenol Reduction Processes
Abstract
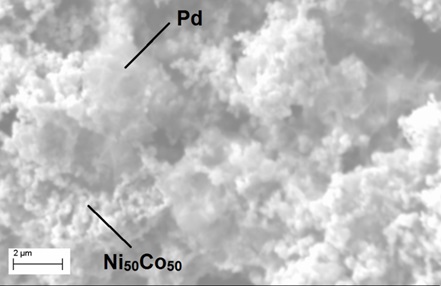
Ni(Co)/Pd nanosized ternary composite materials have been synthesized by the galvanic replacement method. The structure and phase composition of the obtained Ni(Co)/Pd nanostructures were investigated using SEM, EDX and X–ray powder diffraction. The catalytic activity of the synthesized polymetallic Ni(Co)/Pd nanoparticles was studied using the example of the reduction reaction of p–nitrophenol with NaBH4 solution. It was found that in all cases the process of reduction of p–nitrophenol NaBH4 in the presence of Ni(Co)/Pd nanoparticles as a catalyst is described by the first-order kinetic equation for p–nitrophenol. The catalytic activity of the studied nanoparticles based on nickel (Ni) is varied in the following order: Ni < Ni(Co) < Ni(Pd) < Ni(Co)/Pd. It was shown that the decoration of nanoparticles based on d–elements (Ni, Co) with palladium (Pd) significantly increases of their catalytic activity. Moreover, the reduction rate of p–nitrophenol in the presence of Ni(Co)/Pd is almost three times higher, compared to effective catalytic systems known from the references, i. e., such nanosystems can be considered as promising material for the development of new types of magnetically separable catalysts for the production of aminoaromatic compounds.
Downloads
Copyright (c) 2024 Liliya Bazylyak, Pavlo Lyutyy, Vasyl Vynar, Mariana Shepida, Orest Kuntyi, Andriy Kytsya

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Copyright on any article is retained by the author(s).
- The author grants the journal, the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work’s authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal’s published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material is distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





_copy1.png)










