Synthesis of Asymmetric Curcumin Analogue (2,6)-2-(3-bromo-4-methoxybenzylidine)-6-(3,4-dimethoxybenzylidine)cyclohexanone with a Base Catalyst
Abstract
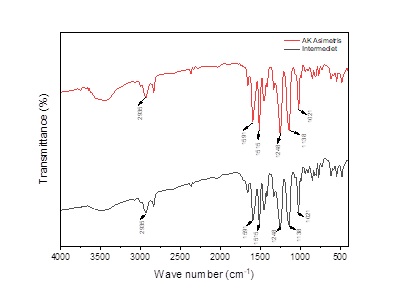
Curcumin analogs are phenolic secondary metabolites that are more stable than curcumin because they do not contain active methylene groups. Generally, these compounds have a symmetric structure, while asymmetric curcumin analogs with higher frequency and potency are rarely synthesized. This study aimed to synthesize asymmetric curcumin analogs from 2-(3,4-dimethoxybenzylidene)cyclohexanone and bromoanisaldehyde. The synthesis was conducted using the Claisen-Schmidt condensation method with a base catalyst in ethanol at 25 °C for 12 hours. The intermediate compound available in the laboratory was characterized using GC-MS, showing a molecular ion (M⁺) at m/z 246. Meanwhile, bromoanisaldehyde was characterized by GC-MS and FT-IR, yielding a molecular ion at m/z 215 and a C–Br stretching vibration peak at 812 cm⁻¹. The study yielded a yellow solid weighing 0.13 g (yield percentage: 2.93%) with a melting point of 143-147 °C. UV-Vis, FT-IR, and HR-MS analysis confirmed the successful synthesis and characterization of the asymmetric curcumin analog, as evidenced by the molecular ion (M⁺) at m/z 443.06274 in the HR-MS spectrum. However, further analysis, such as ¹H-NMR and ¹³C-NMR, is needed to confirm the structure of the compound. Furthermore, research related to bioactivity testing is crucial for obtaining more stable and effective drug candidates.
Downloads
Copyright (c) 2025 Khoirotun Nafillah, Linda Ekawati, Purwanto Purwanto

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Copyright on any article is retained by the author(s).
- The author grants the journal, the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work’s authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal’s published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material is distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





_copy1.png)










