Utilization of Natural Zeolite Impregnated with Fe for Decolorization of Methylene Blue
Abstract
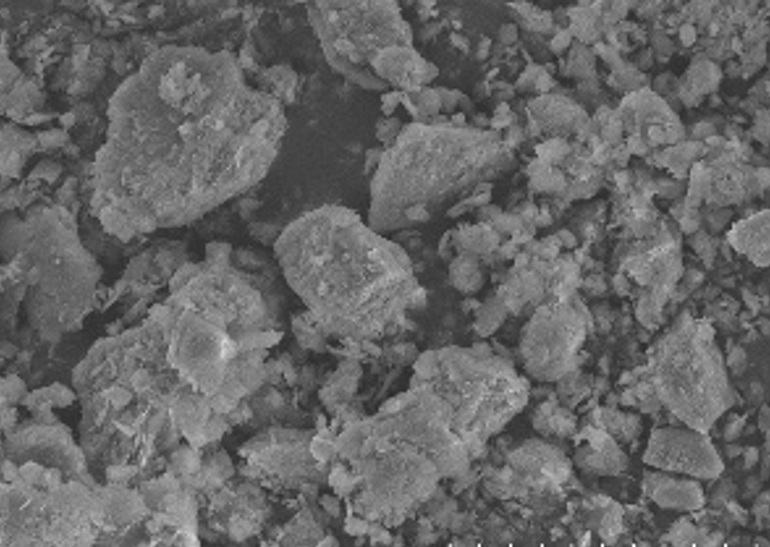
Wastewater containing methylene blue, discharged into rivers, significantly impacts water quality due to its resistance to natural degradation. This study investigated the treatment of methylene blue using the photo-Fenton method, employing UV light to generate hydroxyl radicals (•OH) through the reaction of hydrogen peroxide (H₂O₂) and Fe catalyst. Natural zeolite was used as a support material, activated with NaOH solution, and impregnated with FeSO₄·7H₂O. Semi-quantitative EDS analysis indicated an iron content of 6.2 wt%. The XRD result shows that the crystalline iron phase was hematite. The photo-Fenton experiments were performed at a catalyst dosage of 0.1 g/L to degrade methylene blue with an initial concentration of 20 mg/L by varying pH levels (3, 5, 7) and H₂O₂ concentrations (15, 30, 45 mM). The optimal conditions were found to be a combination of 45 mM H₂O₂ concentration, pH 3, and under 365 nm UV lamp irradiation, achieving a maximum decolorization efficiency of 99.77% at 120 minutes. H₂O₂ concentration did not significantly affect final decolorization percentage, indicating that excess H₂O₂ does not enhance degradation beyond a certain threshold. The lowest final methylene blue concentration achieved was 0.05 mg/L, and the final chemical oxygen demand (COD) was reduced to 243.6 mg/L.
Downloads
Copyright (c) 2026 Tifa Paramitha, Bunga Aulia, Tarisha Aulia Azzahra, Teguh Taufiqurohim, Alfiana Adhitasari

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Copyright on any article is retained by the author(s).
- The author grants the journal, the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work’s authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal’s published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material is distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





_copy1.png)










