Adsorption of Bismarck Brown R Dyes Using Mesoporous Silica MCM-48
Abstract
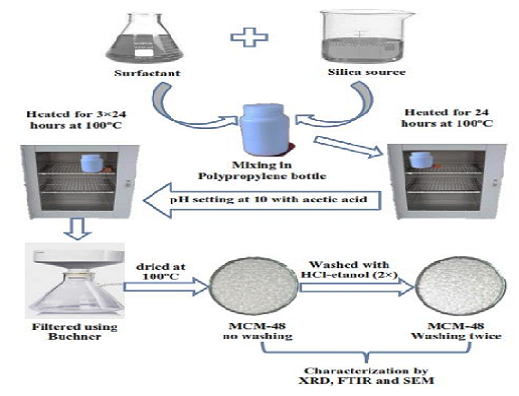
The adsorption of Bismarck Brown R (BBR) dye has been conducted using mesoporous silica (MCM-48). We synthesized the adsorbent using Ludox HS-40 as a silica source and surfactants of Cetyl Trimethylammonium Bromide (CTAB) and Triton X-100. The characterization of MCM-48 was performed using Fourier transform infrared (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM). Various contact times were used to study the adsorption kinetics, and concentrations were used to study the adsorption isotherm. The optimum contact time of Bismarck Brown R dye was 120 minutes, and the adsorption followed a pseudo-second-order model. Based on the equation Langmuir and Freundlich adsorption isotherms, the adsorption capacity values of each are obtained 158.7301 mg g-1 and 4.3601 mg g-1. Our results showed that the material can be used as a new dye adsorbent.
Downloads
Copyright (c) 2022 Muhammad Zakir, Andi Nuraeni, Paulina Taba, Abdul Wahid Wahab, Seniwati Dali, Syaharuddin Kasim, Nursiah La Nafie

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Copyright on any article is retained by the author(s).
- The author grants the journal, the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work’s authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal’s published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material is distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





_copy1.png)










