Characterization and Kinetic Study of Methylene Blue Photocatalytic on ZnO/ZSM-5
Abstract
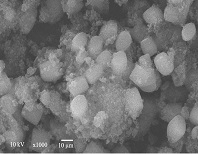
Photodegradation of organic pollutants depends significantly on the structure of metal oxide-based semiconductor photocatalysts. ZnO/ZSM-5 has shown the potential to significantly improve its photocatalytic efficiency for removing waterborne pollutants. ZnO/ZSM-5 has been reported to be an active catalyst for degrading methylene blue. These methods commonly involve various catalytic reactions, with the Langmuir-Hinshelwood process being used to describe the reaction kinetics. A kinetic study on the photocatalytic degradation of methylene blue using ZnO/ZSM-5 was conducted under UV-LED lamp irradiation. ZnO/ZSM-5 was characterized using XRD, SEM, and N2 adsorption-desorption, and it was prepared via the impregnation method. The interaction between ZnO/ZSM-5 and methylene blue solutions over a period of 30 to 180 minutes was monitored using a UV-Vis spectrophotometer. The photocatalytic degradation of methylene blue followed first-order rate kinetics. The Langmuir-Hinshelwood (L-H) kinetic analysis revealed that the photocatalytic reaction constant (kc) was 4.207 L.mg-1. menit-1, and the Langmuir-Hinshelwood constant (K) was 261.509 L.mg-1.
Downloads
Copyright (c) 2023 Hellna Tehubijuluw, Fensia Analda Souhoka, Yuly Kusumawati, Didik Prasetyoko, Riki Subagyo, Reva Edra Nugraha, Aishah Abdul Jalil

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Copyright on any article is retained by the author(s).
- The author grants the journal, the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work’s authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal’s published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material is distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.





_copy1.png)










