SINTESIS ZEOLIT A DARI ABU DASAR BATUBARA (COAL BOTTOM ASH) DENGAN METODE PELEBURAN DAN HIDROTERMAL
Abstract
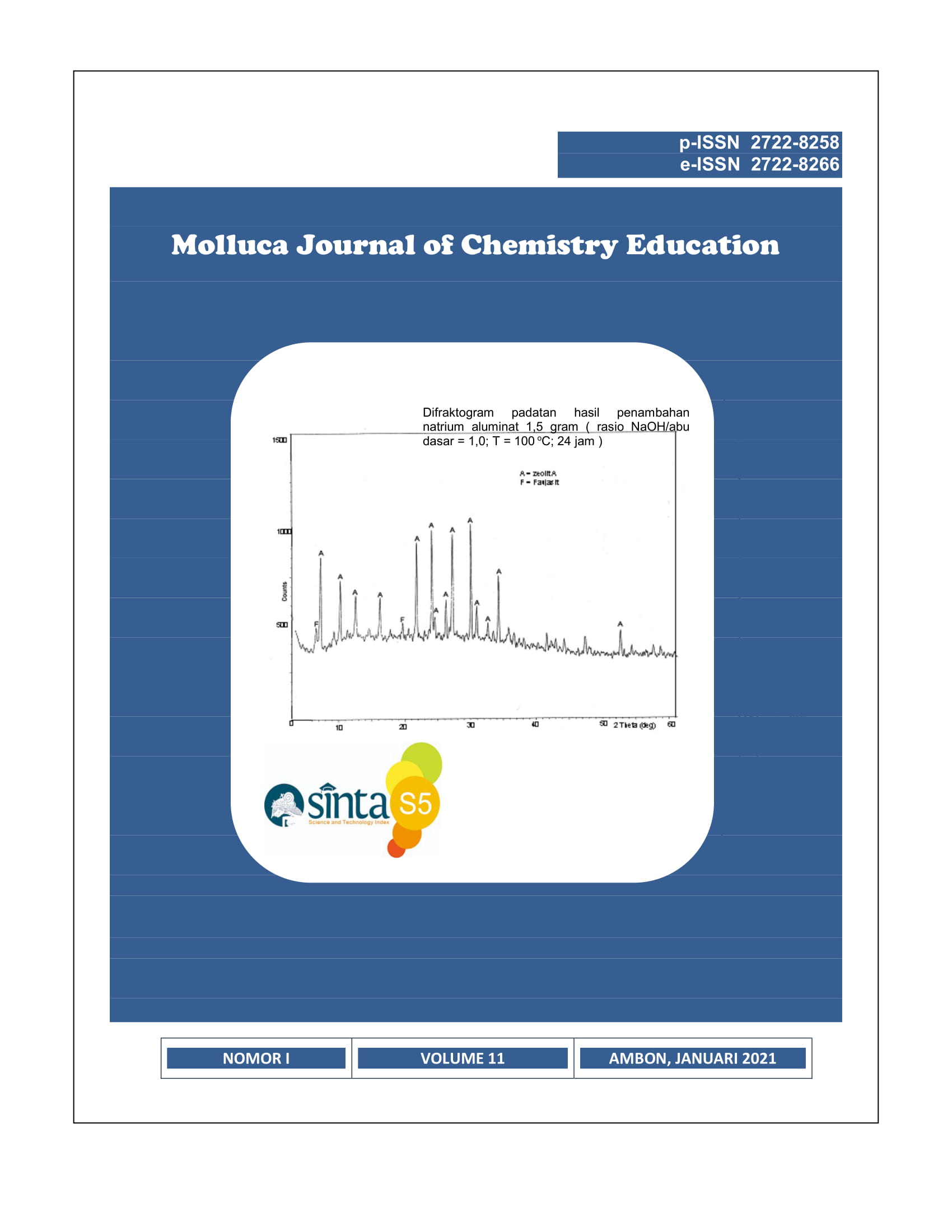
Zeolites can be synthesized from materials containing silica (SiO2) and alumina (Al2O3). The analysis showed that the coal bottom ash from PLTU Paiton contained a number of oxides, namely: SiO2 49.73%; Al2O3 19.51%; Fe2O3 16.18%; CaO 5.40%; MgO 2.96%; Na2O 1.23%; K2O 0.84%; TiO2 0.99%; MnO 0.17%; and LOI of 2.38%. Zeolite from coal bottom ash was carried out by fussion and hydrothermal methods. Based on the percentage of alumina from the bottom ash, it is necessary to add pure alumina for the perfection of the zeolite formed. The addition of 1.5 g sodium aluminate resulted in a higher crystallinity level of the zeolite. This is indicated by the appearance of sharp peaks at 2θ = 30.098 (d = 2.96); 24.14 (d = 3.68); 27.24 (d = 3.27); 6.63 (d = 13.31); 7.08 (d = 12.47); 7.32 (d = 12.06) and 10.30 (d = 8.58). Based on the comparison of the peaks that appear on the diffractogram with the standards proposed by Ballmoos, with the addition of 1.5 grams of aluminate, the overall zeolite formed is zeolite A.
Downloads
References
Layang, Tesis, Universitas Gadjah Mada, Yogyakarta.
Barrer, R.M., 1982, Hydrothermal Chemistry of Zeolite, Academic Press, London.
Hamdan, H., 1992, Introduction to Zeolite: Synthesis, Characterization, and
amodification, University Technology Malaysia.
Izquierdo, M., Vazquez, E., Querol, X., Barra, M., Lopez, A., and Plana, F, 2001,
Use of Bottom Ash from Municipal Solid Waste Incineration as a Road
Material, International Ash utilization Symposium Center for Applied Energy Research, University of Kentucky, Paper # 37.
Kantiranis, N., Georgakopaolos, A., Fillipidis, A., and Drakoulis, A., 2004, Mineralogy ang Organic Matter Content of Bottom Ash samples from Agios Dimitrios Power Plant, Greece, Bulletin of The geologicalSocietyof Greece. Vol XXXVI, 320-326
Penilla, R.P., Bustos, G.A., Elizalde, S.G., 2004, Zeolite Synthesized by Alkaline Hydrothermal Treatment of Bottom Ash from Combustion of 83 Municipal Solid Wastes. J. American ceramic Society, Vol. 86, 1527- 1533.
Querol, X., Moreno, N., Umana, J.C., Alastuey, A., Hernandes, E., Lopez, A., and Plana, F., 2002, Synthesis of Zeolite from coal fly ash: An Overview, International Journal of Coal geology, 50, 413-423.
Ribeiro, R.F., Ridgues, A.E., Rollman, L.D., ang Naccache, C., 1984, Zeolite
Science and Technology, Martinus Nijhoff Publisher, Netherland, 3- 12.
Sighemoto, N., Hayashi, H., and Miyaura, K., 1993, Selective Formation of Na-X, Na-A Zeolite from Coal Fly Ash by Fusion with Sodium Hydroxide Prior to Hydrothermal Reaction, J. Mater. Sci, 28, 4781-4786.
Sighemoto, N., Sugiyama, S., Hayashi, H., and Miyaura, K., 1995, Characterization of Na-X, Na-A and Coal Fly Ash Zeolite and Their Amorphous Precursors by IR, MAS NMR and XPS, J. Mater. Sci, 30, 5777-5782.
Vucinic, D., Miljanovic, I., Rosic,A., and Lazic, P., 2003, Effect of Na2O/SiO 2 mole Ratio on The Crystal Type of Zeolite Synthesized From Coal Fly Ash, J. Serb. Chem. Soc. 68 (6) 471-478.
Copyright (c) 2021 Molluca Journal of Chemistry Education (MJoCE)

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.